Examples
Below are several examples of results obtained utilizing the methods described in the Recommendations and Instructions pages of this site.
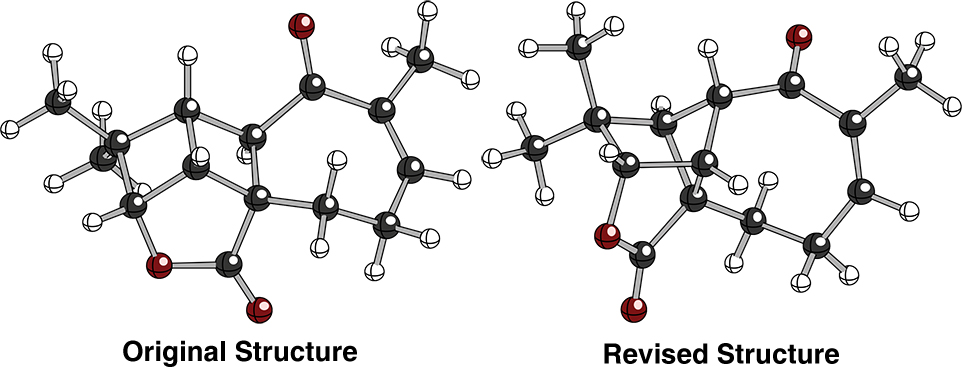
Aquatolide Structure Revision
Computational Method: mPW1PW91/6-311+G(2d,p)-SCRF//B3LYP/6-31+G(d,p)
Aquatolide is a sesquiterpenoid natural product that was originally proposed to have an unusual ladderane core structure.San89a Preliminary NMR calculations on the proposed structure however were not a good match to the reported NMR data. After computationally screening numerous candidate structures on the basis of their NMR shifts, we identified the structure shown below as a likely candidate for the true compound. Our collaborators were then able to re-isolate the original compound and subject it to a host of modern NMR experiments. Careful comparison of the experimental and computed shifts and (unusual) coupling constants strongly supported our proposed revision. Ultimately, a crystal structure was obtained which confirmed the prediction.Tan12b
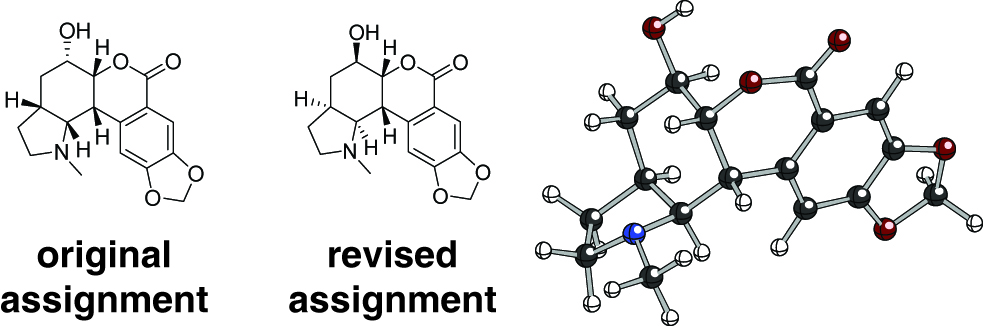
Re-assignment of Nobilisitine A
Computational Method: mPW1PW91/6-311+G(2d,p)-SCRF//B3LYP/6-31+G(d,p)
Nobilisitine A is an alkaloid natural product first reported in 1999.Evi99a In 2010, Banwell et al. synthesized the enantiomer of the reported structure and found significant deviations in the 1H and 13C chemical shifts, particularly for the methyl protons, and noted that an anti relationship of the pyrrolidine and lactone rings was more likely.Ban10a
Lodewyk and Tantillo then computed the chemical shifts for eight diastereomers of the original structure (one of which is the known natural product clividine), and found that while the diastereomers in general had very similar NMR profiles, the one pictured below was the best overall match, considering average deviations and largest outliers.Tan11b In addition, the DP4 statistical method of Smith and GoodmanGoo10a was used for further confidence in this prediction.
Soon after the prediction was made, Banwell, et al. synthesized the predicted revised structure and found that it did indeed match that of the originally-isolated natural product.Ban11a This demonstrates the utility of computed chemical shifts for distinguishing amongst a set of very closely related diastereomers.
Aplydactone - empirical scaling of computed NMR 1H and 13C chemical shifts
Computational Method: mPW1PW91/6-311+G(2d,p)-SCRF//B3LYP/6-31+G(d,p)
Mean Absolute Deviation (1H): 0.08 ppm
Mean Absolute Deviation (13C): 1.5 ppm (does not include the carbon nucleus with the attached bromine atom)
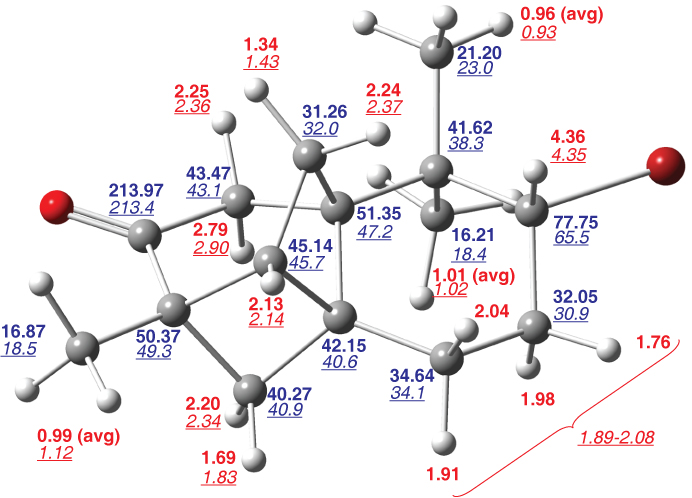
Bold numbers are computed chemical shiftsTanUnp relative to TMS, underlined italic numbers are reported experimental chemical shifts.Sto01a Red values are for 1H and blue values are for 13C.
The same structure calculated at a lower level of theory (B3LYP/6-31+G(d,p)-SCRF//B3LYP/6-31G(d)) results in a mean absolute deviation of 0.11 ppm for 1H and 3.1 ppm for 13C.
Intricarene - empirical scaling of computed NMR 1H and 13C chemical shifts
Computational Method: mPW1PW91/6-311+G(2d,p)-SCRF//B3LYP/6-31+G(d,p)
Mean Absolute Deviation (1H): 0.09 ppm
Mean Absolute Deviation (13C): 1.8 ppm
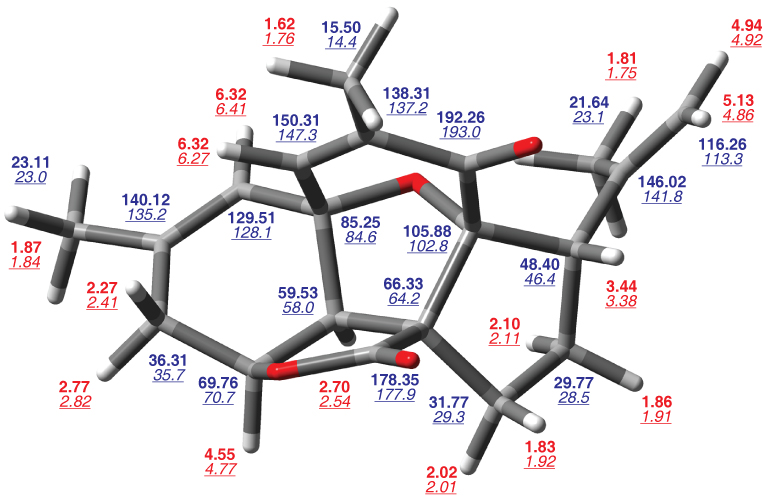
Bold numbers are computed chemical shiftsTanUnp relative to TMS, underlined italic numbers are reported experimental chemical shifts.Rod05a Red values are for 1H and blue values are for 13C.
The same structure calculated at a lower level of theory (B3LYP/6-31+G(d,p)-SCRF//B3LYP/6-31G(d)) results in a mean absolute deviation of 0.09 ppm for 1H and 3.0 ppm for 13C.
Echinopine B - empirical scaling of computed NMR 1H and 13C chemical shifts
Computational Method: mPW1PW91/6-311+G(2d,p)-SCRF//B3LYP/6-31+G(d,p)
Mean Absolute Deviation (1H): 0.08 ppm
Mean Absolute Deviation (13C): 1.9 ppm
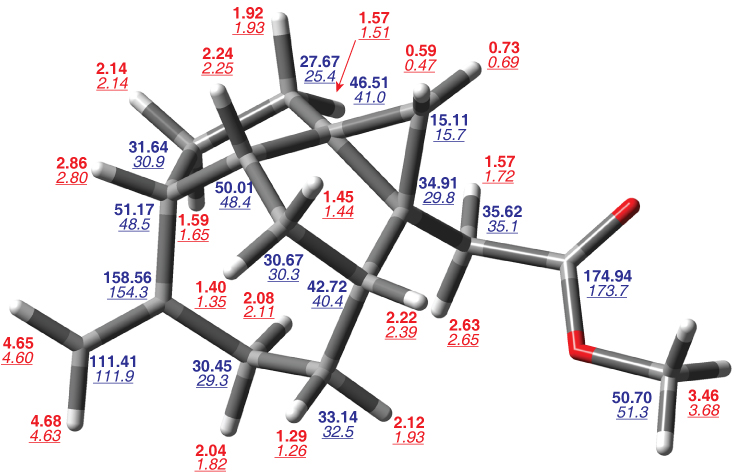
Bold numbers are computed chemical shiftsTanUnp relative to TMS, underlined italic numbers are reported experimental chemical shifts.Shi08a,Mag09a Red values are for 1H and blue values are for 13C. The cyclopropane quaternary carbon experimental assignments are switched relative to those reported.
The same structure calculated at a lower level of theory (B3LYP/6-31+G(d,p)-SCRF//B3LYP/6-31G(d)) results in a mean absolute deviation of 0.10 ppm for 1H and 3.4 ppm for 13C.
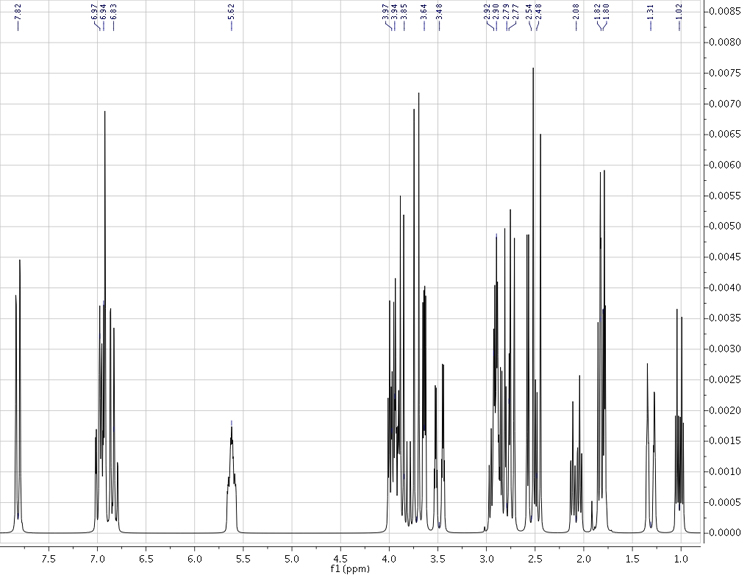
Strychnine - complete simulated 1H spectrum (200 MHz)
Computational Method: WP04/cc-pVDZ//B3LYP/6-31G(d) {nmr=GIAO SCRF(solvent=chloroform)} for chemical shifts
B3LYP/6-31G(d,p)u+1s[H] Fermi contact terms for coupling constants
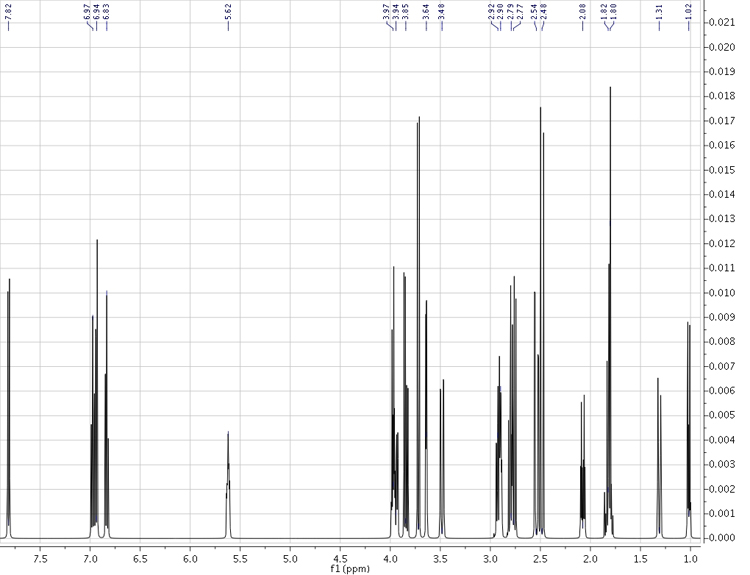
Strychnine - complete simulated 1H spectrum (500 MHz)
Computational Method: WP04/cc-pVDZ//B3LYP/6-31G(d) {nmr=GIAO SCRF(solvent=chloroform)} for chemical shifts
B3LYP/6-31G(d,p)u+1s[H] Fermi contact terms for coupling constants